PhosR Signalomes
Signalomes.RdA function to generate signalomes
Signalomes(KSR, predMatrix, exprsMat, KOI, threskinaseNetwork=0.9,
signalomeCutoff=0.5, module_res = NULL, filter = FALSE, verbose = TRUE)Arguments
- KSR
kinase-substrate relationship scoring results
- predMatrix
output of kinaseSubstratePred function
- exprsMat
a matrix with rows corresponding to phosphosites and columns corresponding to samples
- KOI
a character vector that contains kinases of interest for which expanded signalomes will be generated
- threskinaseNetwork
threshold used to select interconnected kinases for the expanded signalomes
- signalomeCutoff
threshold used to filter kinase-substrate relationships
- module_res
parameter to select number of final modules
- filter
parameter to filter modules with only few proteins
- verbose
Default to
TRUEto show messages during the progress. All messages will be suppressed if set toFALSE
Value
A list of 3 elements.
Signalomes, proteinModules and kinaseSubstrates
Examples
# \donttest{
data('phospho_L6_ratio_pe')
data('SPSs')
data('PhosphoSitePlus')
grps = gsub('_.+', '', colnames(phospho.L6.ratio.pe))
# Construct a design matrix by condition
design = model.matrix(~ grps - 1)
# phosphoproteomics data normalisation using RUV
L6.sites = paste(sapply(GeneSymbol(phospho.L6.ratio.pe), function(x)paste(x)),
";",
sapply(Residue(phospho.L6.ratio.pe), function(x)paste(x)),
sapply(Site(phospho.L6.ratio.pe), function(x)paste(x)),
";", sep = "")
ctl = which(L6.sites %in% SPSs)
phospho.L6.ratio.RUV = RUVphospho(
SummarizedExperiment::assay(phospho.L6.ratio.pe, "Quantification"),
M = design, k = 3, ctl = ctl)
phosphoL6 = phospho.L6.ratio.RUV
# filter for up-regulated phosphosites
phosphoL6.mean <- meanAbundance(phosphoL6, grps = grps)
aov <- matANOVA(mat=phosphoL6, grps=grps)
phosphoL6.reg <- phosphoL6[(aov < 0.05) &
(rowSums(phosphoL6.mean > 0.5) > 0),, drop = FALSE]
L6.phos.std <- standardise(phosphoL6.reg)
idx <- match(rownames(L6.phos.std), rownames(phospho.L6.ratio.pe))
rownames(L6.phos.std) <- L6.sites[idx]
L6.phos.seq <- Sequence(phospho.L6.ratio.pe)[idx]
L6.matrices <- kinaseSubstrateScore(PhosphoSite.mouse, L6.phos.std,
L6.phos.seq, numMotif = 5, numSub = 1)
#> Number of kinases passed motif size filtering: 114
#> Number of kinases passed profile size filtering: 44
#> Scoring phosphosites against kinase motifs:
#> 1.
#> 2.
#> 3.
#> 4.
#> 5.
#> 6.
#> 7.
#> 8.
#> 9.
#> 10.
#> 11.
#> 12.
#> 13.
#> 14.
#> 15.
#> 16.
#> 17.
#> 18.
#> 19.
#> 20.
#> 21.
#> 22.
#> 23.
#> 24.
#> 25.
#> 26.
#> 27.
#> 28.
#> 29.
#> 30.
#> 31.
#> 32.
#> 33.
#> 34.
#> 35.
#> 36.
#> 37.
#> 38.
#> 39.
#> 40.
#> 41.
#> 42.
#> 43.
#> 44.
#> 45.
#> 46.
#> 47.
#> 48.
#> 49.
#> 50.
#> 51.
#> 52.
#> 53.
#> 54.
#> 55.
#> 56.
#> 57.
#> 58.
#> 59.
#> 60.
#> 61.
#> 62.
#> 63.
#> 64.
#> 65.
#> 66.
#> 67.
#> 68.
#> 69.
#> 70.
#> 71.
#> 72.
#> 73.
#> 74.
#> 75.
#> 76.
#> 77.
#> 78.
#> 79.
#> 80.
#> 81.
#> 82.
#> 83.
#> 84.
#> 85.
#> 86.
#> 87.
#> 88.
#> 89.
#> 90.
#> 91.
#> 92.
#> 93.
#> 94.
#> 95.
#> 96.
#> 97.
#> 98.
#> 99.
#> 100.
#> 101.
#> 102.
#> 103.
#> 104.
#> 105.
#> 106.
#> 107.
#> 108.
#> 109.
#> 110.
#> 111.
#> 112.
#> 113.
#> 114.
#> done.
#> Scoring phosphosites against kinase-substrate profiles:
#> done.
#> Generating combined scores for phosphosites
#> by motifs and phospho profiles:
#> done.
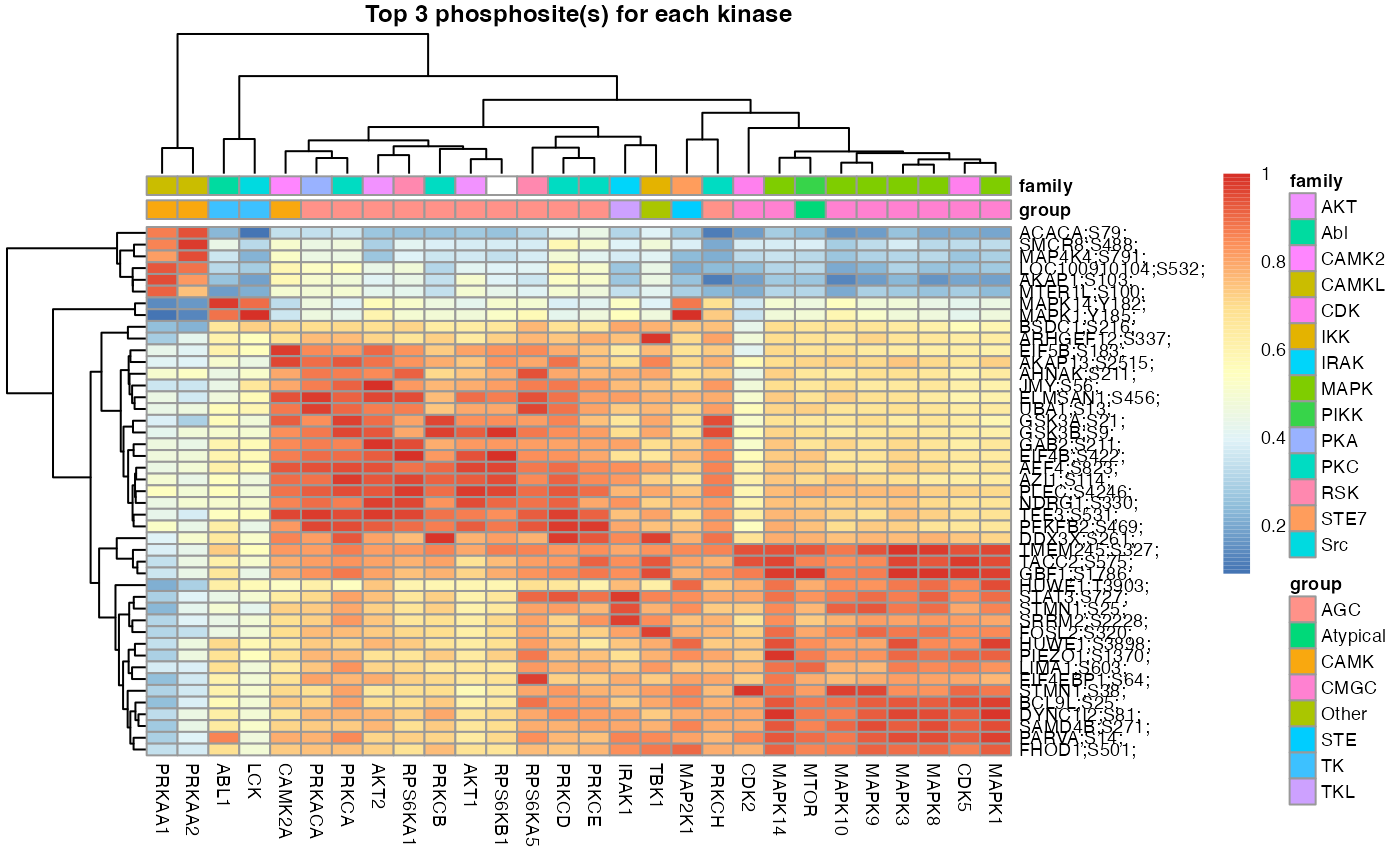 set.seed(1)
L6.predMat <- kinaseSubstratePred(L6.matrices, top=30)
#> Predicting kinases for phosphosites:
#> 1.
#> 2.
#> 3.
#> 4.
#> 5.
#> 6.
#> 7.
#> 8.
#> 9.
#> 10.
#> 11.
#> 12.
#> 13.
#> 14.
#> 15.
#> 16.
#> 17.
#> 18.
#> 19.
#> 20.
#> 21.
#> 22.
#> 23.
#> 24.
#> 25.
#> 26.
#> done
kinaseOI = c('PRKAA1', 'AKT1')
Signalomes_results <- Signalomes(KSR=L6.matrices,
predMatrix=L6.predMat,
exprsMat=L6.phos.std,
KOI=kinaseOI)
#> calculating optimal number of clusters...
#> optimal number of clusters = 3
set.seed(1)
L6.predMat <- kinaseSubstratePred(L6.matrices, top=30)
#> Predicting kinases for phosphosites:
#> 1.
#> 2.
#> 3.
#> 4.
#> 5.
#> 6.
#> 7.
#> 8.
#> 9.
#> 10.
#> 11.
#> 12.
#> 13.
#> 14.
#> 15.
#> 16.
#> 17.
#> 18.
#> 19.
#> 20.
#> 21.
#> 22.
#> 23.
#> 24.
#> 25.
#> 26.
#> done
kinaseOI = c('PRKAA1', 'AKT1')
Signalomes_results <- Signalomes(KSR=L6.matrices,
predMatrix=L6.predMat,
exprsMat=L6.phos.std,
KOI=kinaseOI)
#> calculating optimal number of clusters...
#> optimal number of clusters = 3
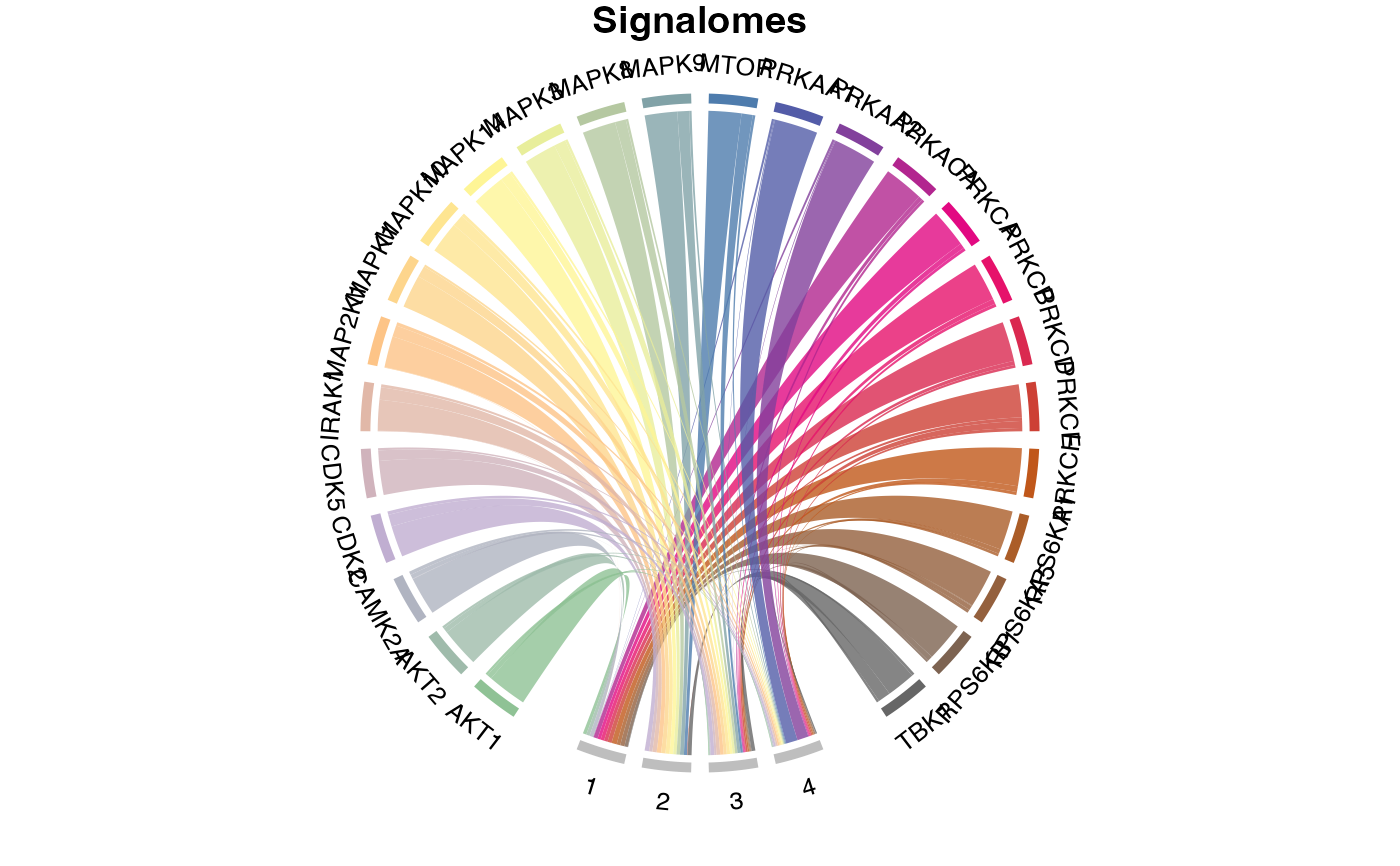 # }
# }